Computational modeling and molecular dynamics simulations of mammalian cytoplasmic tyrosyl-tRNA synthetase and its complexes with substrates
Kravchuk, V. O., Savytskyi, O. V., Odynets, K. O., Mykuliak, V. V., Kornelyuk, A. I.
Abstract. Cytoplasmic tyrosyl-tRNA synthetase (TyrRS) is one of the key enzymes of protein biosynthesis. TyrRSs of pathogenic organisms have gained attention as potential targets for drug development. Identifying structural differences between various TyrRSs will facilitate the development of specific inhibitors for the TyrRSs of pathogenic organisms. However, there is a deficiency in structural data for mammalian cytoplasmic TyrRS in complexes with substrates. In this work, we constructed spatial structure of full-length Bos taurus TyrRS (BtTyrRS) and its complexes with substrates using the set of computational modeling techniques. Special attention was paid to BtTyrRS complexes with substrates [L-tyrosine, K+ and ATP:Mg2+] and intermediate products [tyrosyl-adenylate (Tyr-AMP), K+ and PPi:Mg2+] with the different catalytic loop conformations. In order to analyze their dynamical properties, we performed 100 ns of molecular dynamics (MD) simulations. MD simulations revealed new structural data concerning the tyrosine activation reaction in mammalian TyrRS. Formation of strong interaction between Lys154 and γ-phosphate suggests the additional role of CP1 insertion as an important factor for ATP binding. The presence of a potassium-binding pocket within the active site of mammalian TyrRS compensates the absence of the second lysine in the KMSKS motif. Our data provide new details concerning a role of K+ ions at different stages of the first step of the tyrosylation reaction, including the coordination of substrates and involvement in the PPi releasing. The results of this work suggest that differences between ATP-binding sites of mammalian and bacterial TyrRSs are meaningful and could be exploited in the drug design.
Keywords: aminoacyl-tRNA synthetase; ATP; KMSKS; molecular modeling and dynamics; docking; MolDynGrid
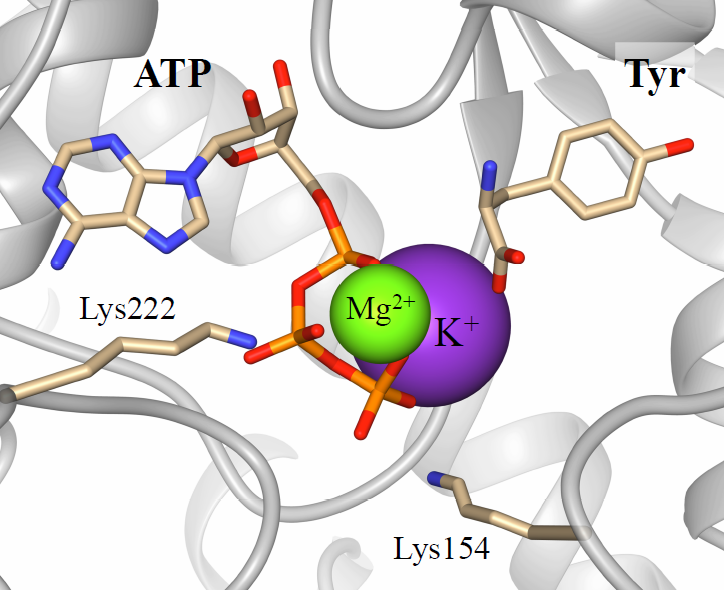
Figure 1. Typical binding of the lysine residues and ATP: Mg2+ into the active site of BtTyrRS. Lys222 (K1) and Lys154 (CP1) bind phosphate groups of ATP in a clump manner. Mg2+ and K+ ions are coordinating the electronegative groups of ATP (triphosphate group) and L-tyrosine (carboxyl group) substrates and bridge them keeping close to each other.
Reference: Kravchuk, V. O., Savytskyi, O. V., Odynets, K. O., Mykuliak, V. V., & Kornelyuk, A. I. (2017). COMPUTATIONAL MODELING AND MOLECULAR DYNAMICS SIMULATIONS OF MAMMALIAN CYTOPLASMIC TYROSYL-tRNA SYNTHETASE AND ITS COMPLEXES WITH SUBSTRATES. J Biomol Struct Dyn., 35(13): 2772-2788. (Impact Factor: 2.919) PDF